来源:深圳翻译公司 更新时间:2014-05-15
[文章摘要]翻译公司招聘人才,出于对翻译服务的考虑,如果觉得译员还满意,就会发一份测试稿给对方做最后的人才判定!可以说,测试稿是翻译公司对译员水平判定的基本标准!
翻译公司常常因业务需要会招聘一些特殊的专业人才,可是怎么辨别那些翻译人才是公司需要的呢?深圳翻译公司给出了招聘医学翻译人才的医学测试稿:
Nonclinical Overview
Overview of the Nonclinical Testing Strategy
The active substance of Maltofer? is an iron(III)-hydroxide polymaltose complex (IPC), a macromolecular complex in which polynuclear ferric hydroxide (pn-Fe(OH)3) is complexed with polysaccharide (oxidised polymaltose) groups. It is highly water soluble over a broad pH range, and unlike simple ferrous salts that are commonly used for oral iron replacement therapy, the complex does not precipitate in an alkaline environment. The molecular weight of the complex is approximately 52,000 Daltons. IPC is currently registered in about 80 countries worldwide for oral iron supplementation, and it has achieved broad acceptance for this route of administration. There is more than 40 years of clinical experience with the product. This overview will consider the available nonclinical safety data that support the use of IPC as an oral iron replacement therapy for the treatment and prophylaxis of iron deficiency states.
The proposed model for the molecular structure of the complex is represented in Figure 2.4-1 below:
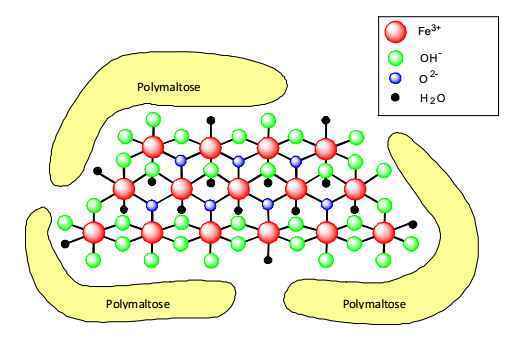
图2.4-1:分子结构模型
Iron(III)-hydroxide polymaltose contains about 28% (mass per mass (m/m)) of iron, equivalent to about 53% (m/m) iron(III)-hydroxide, about 37% (m/m) polymaltose ligand, less than 6.4% (m/m) sodium chloride and less than 10% (m/m) of water. Various presentations of the product are available, including chewable tablets, film-coated tablets, syrups, single-dose containers or drops for oral use. The usual dosage level is in the range 100-300 mg Fe/day in adults (i.e., a maximum of 5 mg Fe/kg/day). In children (age 1-12 years), doses in the range 10-100 mg Fe/day (i.e., up to about 10 mg Fe/kg in a 1-year-old child) may be given depending on the extent of iron deficiency, or whether IPC is being used prophylactically. Treatment may be continued for a few weeks, or even several months when oral iron supplementation is used
prophylactically. The product is used to treat iron deficiency anaemia associated with pregnancy, so may be administered during pregnancy, extending into the post-natal period. IPC is considered to be an optimal iron complex for oral administration, due to its degradation rate and physicochemical characteristics. In this regard, it is as efficient and less toxic than the lower molecular weight iron salts, such as ferrous sulphate or ferrous ascorbate, that have been commonly used to replete the body iron stores by the oral route. As shown in the Clinical Overview (Section 2.5), most ferrous salt preparations induce moderate to severe gastrointestinal (GI) adverse effects, especially nausea, vomiting, abdominal cramps, constipation and diarrhoea, which can in turn result in low compliance with iron therapy. Potential toxicity in case of overdosage is also a risk associated with ferrous salts. In terms of efficiency of iron utilisation, IPC is similar to iron salts, but with a much reduced risk of toxicity occurring in clinical use, or in the overdose situation
Maltofer (IPC) is a specialised pharmaceutical product intended for iron replacement therapy. It possesses no pharmacological activity other than its ability to allow absorption and delivery of utilisable iron to the iron storage and transport proteins in the body (ferritin and transferrin) for the correction of anaemia. For this reason, the value of safety data obtained in healthy, iron-replete animals must be considered to be somewhat limited, since the clinical population will be patients with iron deficiency anaemia. The predicted breakdown products of the IPC complex (iron(III)-hydroxide, maltose, glucose and gluconate) are endogenous molecules. Given this particular set of properties, it was considered reasonable to perform a targeted programme of nonclinical studies on IPC focused on key safety issues.
? Is the complex an efficient means of delivering utilisable iron to the target tissue (i.e., the red blood cell) so as to correct iron deficiency anaemia?
? Are the kinetics and tissue distribution of the iron in the complex similar to that predicted from the behaviour of other iron replacement therapies?
? How does the systemic toxicity profile in animals compare with that produced by iron overload and by other oral iron replacement therapies, in particular iron salts such as ferrous sulphate?
? Are the formulations well tolerated by the oral route of administration, with particular reference to GI tract tolerance?
大家翻译好了可以发过来哦!也可以和我索要优秀的翻译稿哦!
邮箱地址:leo@go-tone.net 或者3243907357@qq.com (私人邮箱)
上一篇:中英文测试稿
下一篇:服装翻译-服装面料英语翻译词汇
|


